Irreversibility
and freezing point depression by
addition of salt
To introduce in an explanation for freezing point depression, at first
a definition of catalysis and anti catalysis is given.
The concept of catalysis is, that an alternative way of reaction is
given, that runs faster than the original one.
But anti catalysis can't work this way. An alternative way, which is
slower, can't stop the original way.
Anti catalysis works in that way, that the original way will be bloced.
So catalysis is a constructive and anti catalysis a destructive
principle.
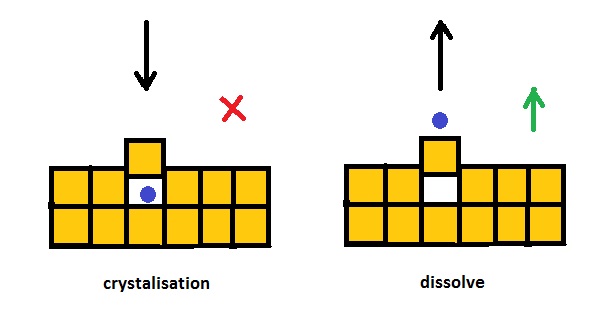
The effect of freezing point depression, caused by addition of salt to
a solution, here is explained with the concept of anti catalysis.
The equilibrium is determined by two contrary prozesses,
crystallisation and dissolution of water.
However an ion of salt can bloc only crystallisation by occupying a
place in the crystall structure. Dissolving remains possible.
And so, by blocing of only one direction of this process, the
equilibrium is shifted and the freezing point drift to a lower
temperature.
But what has this to do with the concept of irreversibility ? In the
original sense of irreversibility, the phenomeneon is described as
catalysis, which opens only one direction of a process. But the same
result is possible, if only one way is bloced.
Homeopathy- not as stupid as you think !
